Cancer is a mysterious disease for many reasons. Chief among the unknowns are how and why tumors form.
Two University of Iowa studies offer key insights by recording in real time, and in 3–D, the movements of cancerous human breast tissue cells. It's believed to be the first time cancer cells' motion and accretion into tumors has been continuously tracked. (See accompanying videos.)
The team discovered that cancerous cells actively recruit healthy cells into tumors by extending a cable of sorts to grab their neighbors—both cancerous and healthy—and reel them in. Moreover, the Iowa researchers report that as little as five percent of cancerous cells are needed to form the tumors, a ratio that heretofore had been unknown.
“It’s not like things sticking to each other,” said David Soll, biology professor at the UI and corresponding author on the paper, published in the American Journal of Cancer Research. “It’s that these cells go out and actively recruit. It’s complicated stuff, and it’s not passive. No one had a clue that there were specialized cells in this process, and that it’s a small number that pulls all the rest in.”
The findings could lead to a more precise identification of tumorigenic cells (those that form tumors) and testing which antibodies would be best equipped to eliminate them. Soll's Monoclonal Antibody Research Institute and the Developmental Studies Hybridoma Bank, created by the National Institutes of Health as a national resource, directed by Soll and housed at the UI, together contain one of the world’s largest collections of antibodies that could be used for the anti-cancer testing, based on the new findings.
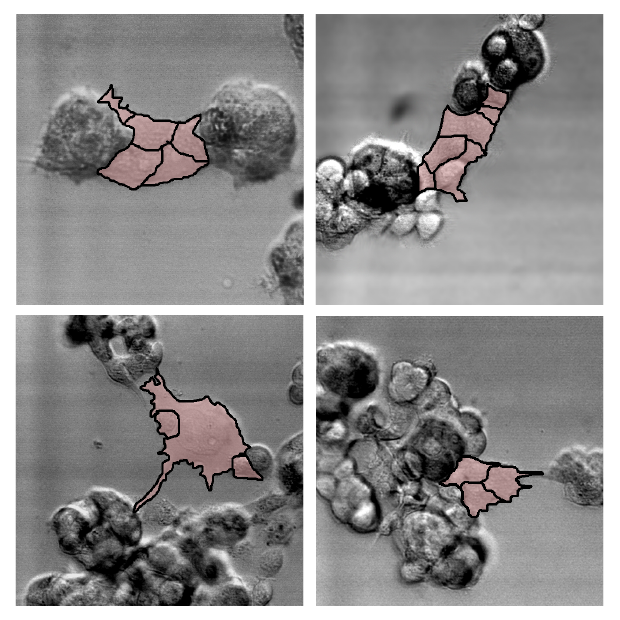
In a paper published last spring in the journal PLOS One, Soll’s team showed that only cancerous cells (from a variety of cancers, including lung, skin, and aggressive brain tumors known as glioblastomas) engaged in tumor formation by actively soliciting other cells. Like evil-minded envoys, individual cancer cells extend themselves outward from the original cluster, probing for other cells in the area, the researchers observed. Once it detects one, the extended cell latches on and pulls it in, forming a larger mass. The activity continues, the cancerous extensions drawing in more and more cells—including healthy cells—as the tumor enlarges.
“There’s nothing but tumorigenic cells in the bridge (between cells),” Soll said, “and that’s the discovery. The tumorigenic cells know what they’re doing. They make tumors.”
The question is how these cells know what to do. Soll hypothesizes they’re reaching back to a primitive past, when these cells were programmed to form embryos. If true, perhaps the cancerous cells—masquerading as embryo-forming cells—recruit other cells to make tissue that then forms the layered, self-sustaining architecture needed for a tumor to form and thrive.
Think of a Death Star that’s built up enough defenses to ward off repeated attacks. Or, less figuratively, how bacteria can conspire to create an impenetrable film on surfaces, from orthopedic implants to catheters.
“There must be a reason,” Soll said. “You might want one big tumor capable of producing the tissue it needs to form a micro-environment. It’s as if it’s building its own defenses against the body’s efforts to defeat them.”
In the AJCR paper, the researchers compared the actions of human breast tissue cells (MoVi-10') to a weakly tumorigenic, parental breast cancer cell line (MCF-7). First, they found that over a 50-hour period, MoVi-10'–only cells grew more in density, primarily by joining together, than did MCF-7.
Also, in all instances, regardless of the ratio of MCF-7 to MoVi-10' cells in the cluster, only MoVi-10' cells reached out and drew in other cells—including healthy cells—to the growing mass.
“The results here extend our original observation that tumorigenic cell lines and fresh tumor cells possess the unique capacity to undergo coalescence through the active formation of cellular cables,” the authors write.
The finding lends more weight to the idea that tumors are created concurrently, in multiple locations, by individual clusters of cells that employ the cancer-cell cables to draw in more cells and enlarge themselves. Some have argued that tumors come about more by cellular changes within the masses, known as the “cancer stem cell theory.”
Soll’s team also discovered that the Mo-Vi10' cells move at 92 microns per hour, about twice the speed of healthy cells. That’s important because it helps scientists better understand how quickly tumors can be created.
Contributing authors, all from the University of Iowa, include Joseph Ambrose, Michelle Livitz, Deborah Wessels, Spencer Kuhl, Daniel Lusche, and Edward Voss. Amanda Scherer, now at the University of Michigan, also contributed to the research while at the UI.
The Developmental Studies Hybridoma Bank funded the study.